Articles
Revolutionizing Early-Stage Biopharma Development: Introducing Wheeler Bio’s Portable CMC® Platform
January 2024
Please fill out the form below for more information about Wheeler’s Lead-to-Clinic services
"*" indicates required fields
Abstract:
Speeding the drug development process is imperative for the industry to improve healthcare outcomes and drug accessibility. Not surprisingly, accelerated approvals have become the norm over the last decade (over two-thirds of drug approvals in 2020 followed FDA Fast Track, Breakthrough Therapy, Priority Review, or Accelerated Approval approaches). However, pressure to accelerate often correlates with insufficient time reserved for chemistry, manufacturing, and controls (CMC), which accounts for significant risk. At Wheeler Bio, we believe that, by integrating the translational steps between discovery and manufacturing using a well-characterized CMC platform, we can enhance the capital efficiency of pre- and post-IND pipeline management for our clients by keeping CMC off the critical path to first-in-human (FIH) trials and future trials. Given the warming of the biopharma funding environment, outsourcing providers have an opportunity to rethink the early clinical material supply chain to support client goals. With our Portable CMC® platform for antibody products, Wheeler Bio is helping start-up biopharma companies engage high-quality, purpose-built manufacturing resources in a capital-calibrated fashion, offering a step change in reducing life cycle CMC burn, CMC technical risk, and CMC regulatory risk.
New Approach to Translation from Discovery to CMC Development Needed
Although the druggable target universe continues to expand for biopharmaceutical developers, R&D costs are rising at a time when downward pricing pressures are also increasing, and expectations are heightened for measurably improved efficacy for new products compared with those already on the market. Being first to market with cost-effective, highly differentiated medicines is imperative to the success of not only the individual programs but the biopharma developers themselves.
Simultaneously, developers inherently face huge risks when translating drug candidates into FIH clinical studies. Developers typically spend $3–8 million in seed money at the discovery stage, $15–40 million in Series A funding for FIH studies, and $60–100 million in Series B funding for later-stage clinical trials. Spending on outsourcing is minimal during discovery but increases once candidates enter FIH studies.
For smaller biopharma companies, between the discovery and FIH study stages of development, there is often a period of time during which seed money has run out and Series A funding has not yet become available, yet the burn rate during that period may average around $150,000 per month. Contract development and manufacturing organizations (CDMOs) do not typically share the risks associated with translation from discovery to CMC development, which leaves the innovator company especially vulnerable to the consequences of failed or inefficient CMC efforts. In addition, when strategizing ahead of fundraising efforts, many drug developers do not fully appreciate the value that early CMC data can have in attracting funding and fail to appropriately time their efforts to enable them to present those data when they can be most critical.
Minimizing the translation gap can greatly increase the likelihood of a drug candidate advancing into FIH studies and finding success in later-stage clinical trials. Consequently, drug developers have an acute need to change the status quo and establish a new approach to drive the translation from discovery to CMC development and to enable greater and more comprehensive utilization of early CMC data during fundraising efforts.
Faster evaluation of drug candidates facilitates more rapid elimination of candidates with no potential to advance (“failing fast”), contributing to lower costs and accelerated progression of candidates with greater potential to the clinic. Furthermore, increasing the speed at which programs advance from discovery to FIH studies while still ensuring the safety, identity, strength, purity, or quality (SISPQ) of investigational drug candidates can help mitigate risks for investors by decreasing the likelihood of program failure due to cost overruns and minimizing potential impacts to patient access.
New technologies and/or approaches must be developed and implemented to overcome the bottlenecks associated with the current paradigm of biologic product development. Such solutions must accelerate development efforts without compromising quality by eliminating any unnecessary time-consuming and expensive activities from the critical development path. They must also be sufficiently flexible to allow developers to effectively navigate the translational space, efficiently achieve CMC milestones, and successfully raise funds in a timely manner.
Introducing Portable CMC®
With the implementation of its Portable CMC® platform for antibody products, Wheeler Bio is helping start-up and emerging biopharma companies reduce manufacturing risks in parallel with drug discovery to enable them to progress smoothly and successfully into drug development and FIH studies. Portable CMC® allows standardization of drug development around open-source manufacturing processes and parallel integration between service providers to streamline tech transfer, simplifying the pathway between drug discovery and scalable clinical biomanufacturing.
Five value attributes serve as the foundation of the Portable CMC® platform. They include funding alignment, accessibility, affordability, process freedom, and speed-to-clinic. Modular work packages can be aligned to the specific product development and fundraising milestones of each individual customer, while the predictable scope with all-inclusive pricing combined with no need for large upfront financial commitments significantly reduces the risk for innovators. The use of open-source, client-owned processes, meanwhile, de-risks scale-up and tech transfer. Access to new technologies backed by solid proof-of-concept data, such as stable bulk cultures (SBCs), helps rapidly progress innovators through CMC milestones.
Underlying the development of the Portable CMC® platform is Wheeler Bio’s commitment to efficiency and data-driven decision making and focus on achieving scalability and flexibility for commercial viability. Wheeler’s tech stack of preclinical and clinical development services based around discrete CMC milestones was designed to empower innovators by reducing the risks and costs involved in bringing monoclonal antibody therapeutics from discovery to the clinic.
Understanding Portable CMC®
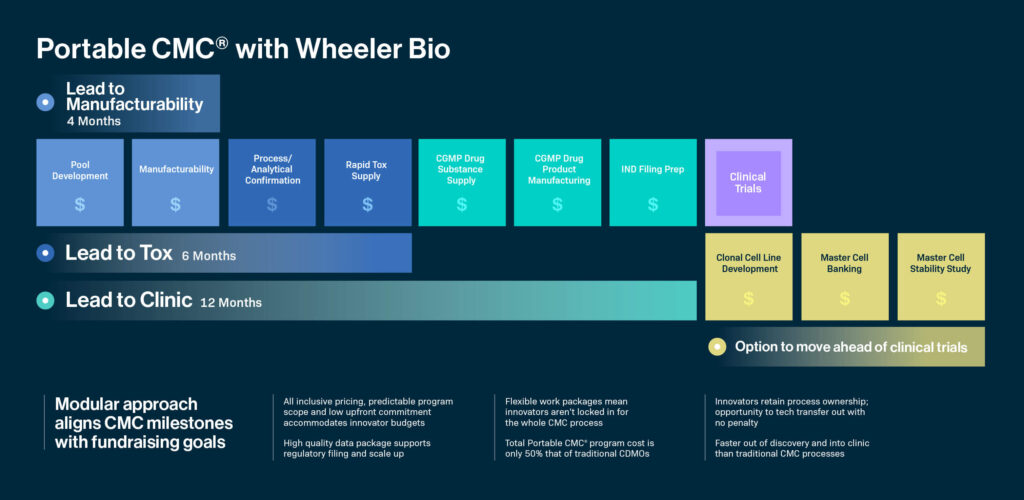
The Portable CMC® platform comprises three core modules: Lead to Manufacturability, Lead to Tox, and Lead to Clinic.
Lead to Manufacturability offers a streamlined approach to generating representative material for early product quality assessments and manufacturability and developability evaluations during drug discovery. Utilizing transposon-mediated gene delivery tools, Wheeler efficiently produces high-yield non-clonal cell lines with consistent product quality by scaling CHO pools for multiple antibody candidates, assessing product quality attributes, delivering purified materials, conducting formulation screening, and establishing stable pools for single-cell cloning. The resulting data aid in selecting top candidates for further CMC development and clinical manufacturing while also providing preclinical materials in milligram quantities (50 mg of protein A–purified material for four or more mAb candidates). Wheeler’s high-productivity cell lines and transposon-based gene delivery reduce quality risks and facilitate a smooth transition from lead selection to clinical supply manufacturing, ultimately accelerating CLD activities by months.
Lead to Tox provides a rapid and accessible method to generate representative materials for toxicology studies, utilizing product quality data and manufacturability assessments to identify the top product candidate. Once selected from manufacturability studies, the Wheeler process platform scales up to 40 L or even 500 L for product supply. Lead to Tox encompasses CHO pool expression for multiple antibody candidates, product quality attribute evaluations, formulation screening, and generation of Protein A–purified drug substance for non-GMP toxicology studies. Leveraging CHO pools and the Portable CMC® process and analytical platforms, Lead to Tox expedites material supply for toxicology studies, ensuring these studies do not become a bottleneck on the path to clinical trials and potentially unlocking further funding opportunities.
Lead to Clinic comprises a streamlined path from cell line development (CLD) to IND submission, providing consistent CGMP production runs for clinical drug substance supply at scales ranging from 50 L to 500 L, along with drug product filling, QC testing, and release. Lead to Clinic furnishes a large set of deliverables, including Master Cell Banks (MCBs) or SBC banks ready for single-cell cloning; 50–500 g of purified drug substance; analytical method development and qualification, and formulation development and manufacturability assessment, along with full process definition and CGMP transfer packages. This comprehensive offering accelerates toxicology studies, aids in IND filing, and allows for flexible tech transfer to other CDMOs if desired, promoting a seamless transition from early development to commercial manufacturing. Lead to Clinic also presents the option to defer clonal development and master cell banking until the program has entered the clinic, which can potentially save critical time and resources until the unlocking of further funding milestones.
Throughout this process, open-source collaboration enables the use of standard, robust manufacturing and automation technologies integrated with digital solutions, such as off-the-shelf, artificial intelligence (AI)-powered process development products. This approach is combined with Wheeler Bio’s well-characterized, quality by design (QbD)-based platform for drug substance manufacturing and testing, resulting in a flexible and streamlined approach to CMC development that can be tailored to each customer’s program milestones, budget, and fundraising timelines.
By accessing manufacturing earlier and at lower cost, customers can more quickly evaluate the developability and manufacturability of candidates and thereby fail faster with unlikely candidates and bring forward those with the greatest chance for success. Overall, providing a more predictable path between drug discovery and IND filing can reduce clinical timelines significantly, which can make the difference between a program’s failure and success.
Advantages of Portable CMC® for Early Development and Beyond
There are several important benefits of the Portable CMC® platform, particularly for smaller biotechs with limited resources and knowledge of the drug development cycle and its evolving regulatory requirements. In general, they include ease of access with low-cost entry and simple service terms for lead selection –– combined with an increased predictability of cash burn owing to the all-in-one pricing approach. Direct assessment of product quality attributes and manufacturability in a stable expression system early in the development cycle reduces the risk associated with lead candidate selection, while our platform approaches and use of SBCs and rapid tox material supply together reduce the time to the clinic.
Overall, the Portable CMC® platform enables seamless translation from discovery to scalable manufacturing (gene-to-GMP) with greater speed, reduced risk, flexibility, predictability, and affordability. Furthermore, each set of services is thoughtfully aligned to match typical funding sources, timing. and milestones.
Asynchronous Programming: The Key to Rapid Material Generation
Biotech start-ups do not only need CDMOs to offer efficient platforms; they also need access to their supporting data sets to facilitate risk assessment and regulatory filings. SBCs make that possible. They enable both rapid material generation and asynchronous workflows so that innovators can benefit from access to early tox data and reduced time to FIH studies.
Well-controlled SBCs are derived from animal cells like Chinese hamster ovary (CHO) cells using specialized recombineering tools and comprise homogeneous cell pools characterized by high gene-copy numbers and limited phenotypic diversity. The homogeneity of SBCs means that the screening effort to identify a comparable clone is significantly reduced. Furthermore, with the tools and technologies available today, the expression of recombinant therapeutic proteins in SBCs for use as toxicology supplies and early clinical development materials is a practical means to dramatically accelerate new drug development efforts without compromising quality, efficacy, or safety.
Timeline Difference: Portable vs. Traditional CMC
The typical development process with a traditional CDMO starts with clone development, which is followed by quality and manufacturability assessments. Next comes cell banking, tox supply, and the production of clinical trial material. This process usually takes about 18 months.
Many developers spend six months or more winnowing down a large set of possible candidates to the top few that are then advanced. They also must make a huge upfront commitment and are typically then locked into using just one CDMO for the entire development cycle. In addition, the typical fee-for-service approach allows for changes to the project scope and associated budget creep, both of which happen frequently. Equally frustrating, time and cost must be sunk into clone development early on with access to only minimal lead viability data, and the CDMO ends up owning the process, resulting in the need to proceed to GMP manufacturing with that CDMO or face significant hurdles and delays.
At Wheeler Bio, both the time and cost of development and CMC risks are dramatically reduced through our Portable CMC® platform. The full platform goes beyond the production of clinical trial material to include cell-line development, master cell banking and master cell stability studies, all of which are completed within the same 18 months required for traditional CDMOs to provide clinical trial material. Notably, the overall program cost is approximately half that of programs implemented by traditional CDMOs.
Significant time and cost reduction is achieved by leveraging SBC pool generation technology. Access to data early on helps us not only choose the top candidate more quickly but to do so in time to support fundraising activities. In addition, despite the shorter timeline, the data packages we generate are of the highest quality and comprehensively support regulatory filing and scale-up. Furthermore, innovators retain process ownership, enabling tech transfer to other CDMOs without any penalties.
In fact, with the Portable CMC® platform, one lead molecule can be selected in just four months using CHO pool cultures (lead to manufacturability assessment). In addition, from the time of the first CHO pool expression, tox material supply can be achieved in just six months (lead-to-tox), and IND filing can be realized in just 12 months (lead-to-clinic).
Infographic: Visualizing the Impact of Portable CMC®
Cutting-Edge Technologies and Partnerships Powering Portable CMC®
The success of Portable CMC® is enabled by Wheeler Bio’s dedication to embracing innovative technology and advancing biopharma development and manufacturing toward goals of the Pharma 4,0 paradigm. The integration of next-generation data-driven and automated platforms into the Portable CMC® offering through the cultivation of key strategic partnerships has been central to Wheeler’s vision of disrupting the biopharma status quo and establish a new, better model. Among our valued partners are ATUM, whose Leap-In Transposase® allows the efficient generation of stable expression cell lines with reduced variability and timelines; DataHow, whose AI- and machine learning–driven data science platform is accelerating process development and intensification; Ambr®, who provides advanced minibioreactor systems (Ambr®15 and Ambr®250) that modernize workflows in clone selection, process optimization, and scale-up; Solentim, whose Ecosystem unifies a suite of instruments (VIPS™, Cell Metric®, and ICON™) and a data management system (STUDIUS™) to streamline CLD, and Charles River Laboratories, who provide us with their RightSourceSM insourced QC testing suite, ensuring effective QC activities while allowing us to focus our efforts on innovation.
Revolutionizing Biotech Development with Portable CMC®
Establishing a new and reliable means of being first to market with new biologic drug products requires a transformative change to accelerate the development timeline. Accomplishing that goal, however, must be achieved while assuring the safety and efficacy of drug candidates and generating sufficient data to attract appropriate levels of funding and support IND filing.
Wheeler Bio is an agile, boutique CDMO inside a venture studio focused on helping small and emerging biotechs eliminate the translation gap from discovery to FIH studies by reducing the cost and time of antibody drug development. We encourage biotech innovators to embrace a data-driven, efficient, and scalable approach, such as that afforded by our Portable CMC® platform.
The platform supports innovators from discovery to GMP manufacture (CMC) with an open-source playbook that is technology-stacked. Three core services — Lead to Manufacturability, Lead to Tox, and Lead to Clinic — are designed to add value through funding alignment, accessibility, affordability, process freedom, and speed to clinic.
Our comprehensive CMC development and manufacturing services are well integrated with digital solutions and include the generation of stable pools and clones; cell substrate testing; process development, optimization, intensification, and characterization; analytical method development, optimization, and qualification; formulation development; preclinical material supply from pools or clones; and CGMP cell banking and production of clinical trial materials.
All these services are provided from state-of-the-art development and manufacturing centers in Oklahoma City, Oklahoma, that are closely linked. The ability to move rapidly from development to production supported by collaborative teams comprising all aspects of both development and operations is a game changer in terms of the speed and process economics that Wheeler Bio offers. Extensive digitalization and automation further reduce risk while boosting productivity and efficiency across all activities.
About Wheeler Bio
Wheeler Bio is a biomanufacturing pioneer founded by a team of industry experts and strategic investors who believe a different CDMO model is needed to help innovators reach their clinical milestones faster. Wheeler Bio’s technology platform, Portable CMC®, simplifies the path between drug discovery and clinical manufacturing by providing a new bridge for translating discoveries to first-in-human trials through increased momentum during technology transfer, shorter timelines, reduced risk, and lower costs. Wheeler’s novel hub-and-spoke operational model, centered in the biomanufacturing metro of Oklahoma City, and integrated with biotechs and discovery CROs, will revolutionize the speed of drug development.