Blogs
Analyzing Bioreactor Sizes from Lab to Clinical Production
September 2024
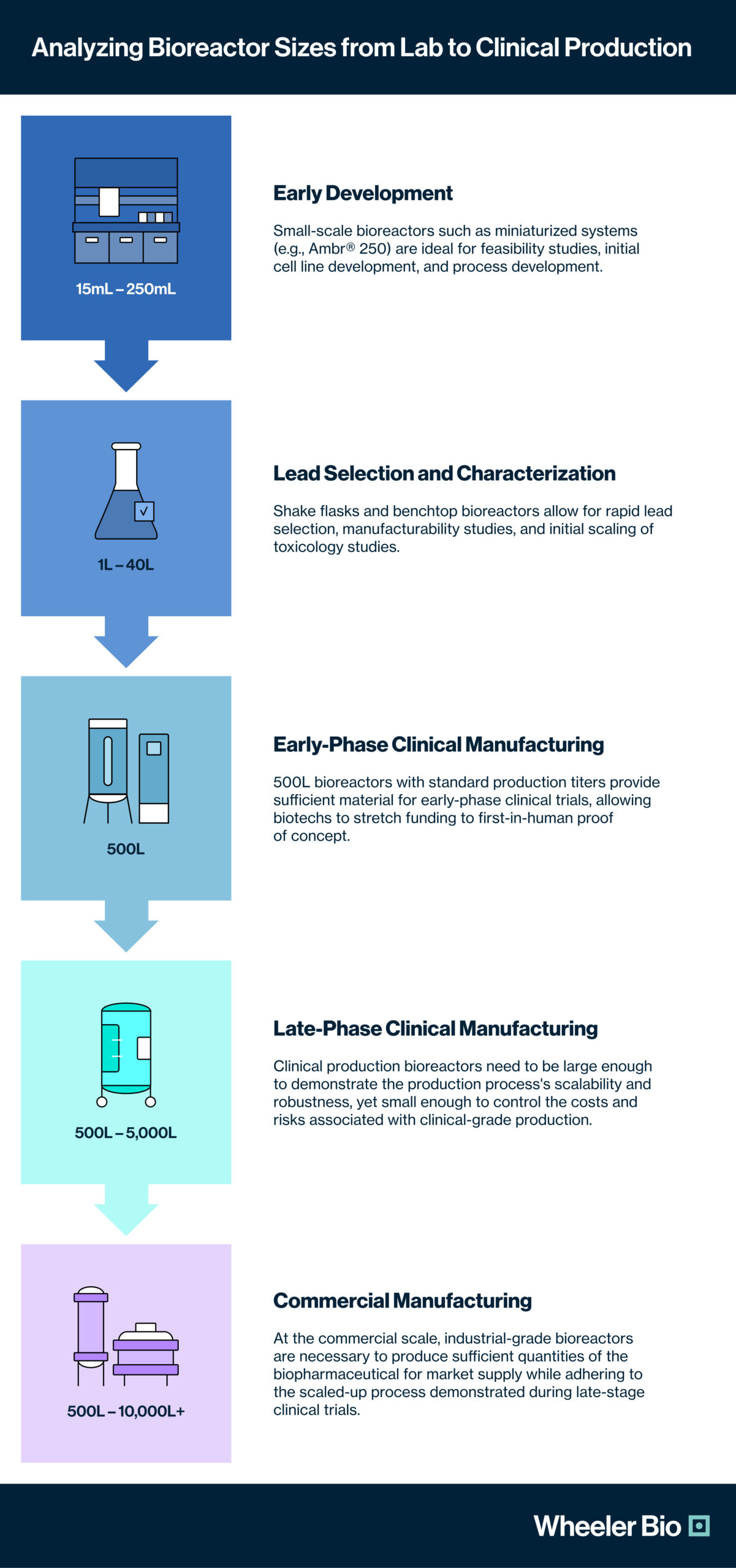
Understanding and planning the bioreactor scale-up process is crucial for a biotech innovator to develop and manufacture a biopharmaceutical product successfully. Each stage of development must be aligned with the right bioreactor size to ensure efficiency, cost-effectiveness, and regulatory compliance. Large CDMO partners often require their biotech clients to follow production programs using 2000L+ reactor systems to scale their lead candidates for first-in-human use, which is far more material than is necessary for phase I clinical trials. Wheeler Bio utilizes a “right-scale” approach to CGMP manufacturing that marries modern, rapid cell line development techniques with lower-volume bioreactors for the most reliable clinical production, aligning to their customer’s needs for accelerating speed to clinic while reducing costs.
Below is an analysis of the optimal bioreactor sizes for each stage of the development and manufacturing journey: from early preclinical development through lead selection and lead characterization, to materials scale-up for toxicology studies, to clinical manufacturing for early and late-phase clinical trials, and finally, to commercial manufacturing of a market-ready therapeutic.