Blogs
An agile approach streamlines the path to clinic for novel monoclonal antibodies
September 2023
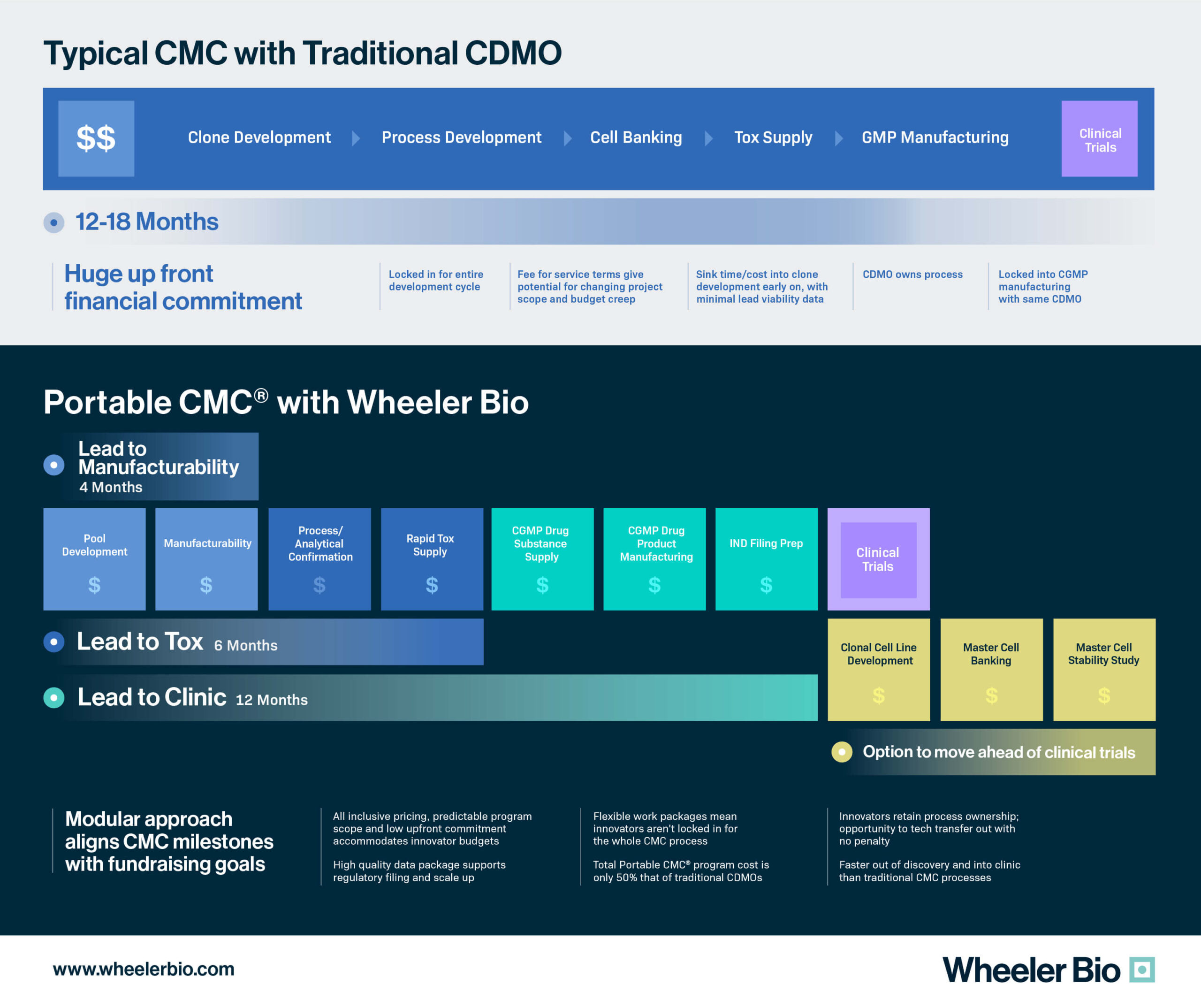
The path from gene to first-in-human is anything but a simple one for virtual and small biotechs. From lead molecule selection to defining the early clinical CMC strategy, there are bottlenecks, regulatory challenges, and a measure of unpredictability that impose significant financial risk to sponsors. By leveraging established manufacturing and analytical platforms that approach early clinical CMC using non-clonal cells (i.e., deferred cloning), sponsors can reduce financial risk without compromising assurances of safety, quality, and regulatory compliance.
This approach is fully embodied in Wheeler’s Portable CMC®. Our platform was purpose-built for biotechs to better align with early-phase fundraising schedules. Portable CMC® is a paradigm shift in CDMO offerings and enables ease of access to CMC services, rapid supply of preclinical and clinical materials, rapid qualification of DS release methods, and an open-source CMC package. Overall, Portable CMC® aims to create a path to first-in-human that is more agile, flexible, rapid, and cost-effective for today’s developers of next-gen products.