Blogs
5 Techniques for Recombinant Protein Expression
May 2024
Exploring Methods of Recombinant Protein Expression with Wheeler Bio
Recombinant protein expression and purification have revolutionized biotechnology by enabling mass production of therapeutic proteins such as insulin, growth factors, and monoclonal antibodies. This technology plays a vital role for developing targeted treatments for cancer and autoimmune disorders, vaccines, and providing enzymes for replacement therapies in genetic disorders. Additionally, recombinant proteins are essential for diagnostic tests, advancing personalized medicine, and producing biosimilars to enhance accessibility and affordability of treatments. When contemplating upstream and downstream protein production, the choice of protein expression system depends on various factors, including the specific protein being produced, desired protein attributes and characteristics, anticipated production scale, and downstream purification scheme. Selecting the appropriate expression system is contingent on the characteristics and intended application and can be crucial to be able to generate adequate quantities of the protein at reasonable costs.
Wheeler Bio offers both transient and stable mammalian protein expression systems that can deliver rapid and cost-effective material generation, which is critical when establishing proof-of-concept on tight timelines. For transient production (CHO and HEK), we utilize the Expi expression systems. For stable production, our CHO-based protein production platform utilizes ATUM’s Leap-In Transposase® technology to rapidly produce milligram-to-gram quantities of recombinant proteins. To learn more about how Wheeler Bio facilitates the complex journey from innovative drug discovery to market release, read our latest article.
Below are the different methods that are used to produce recombinant proteins.
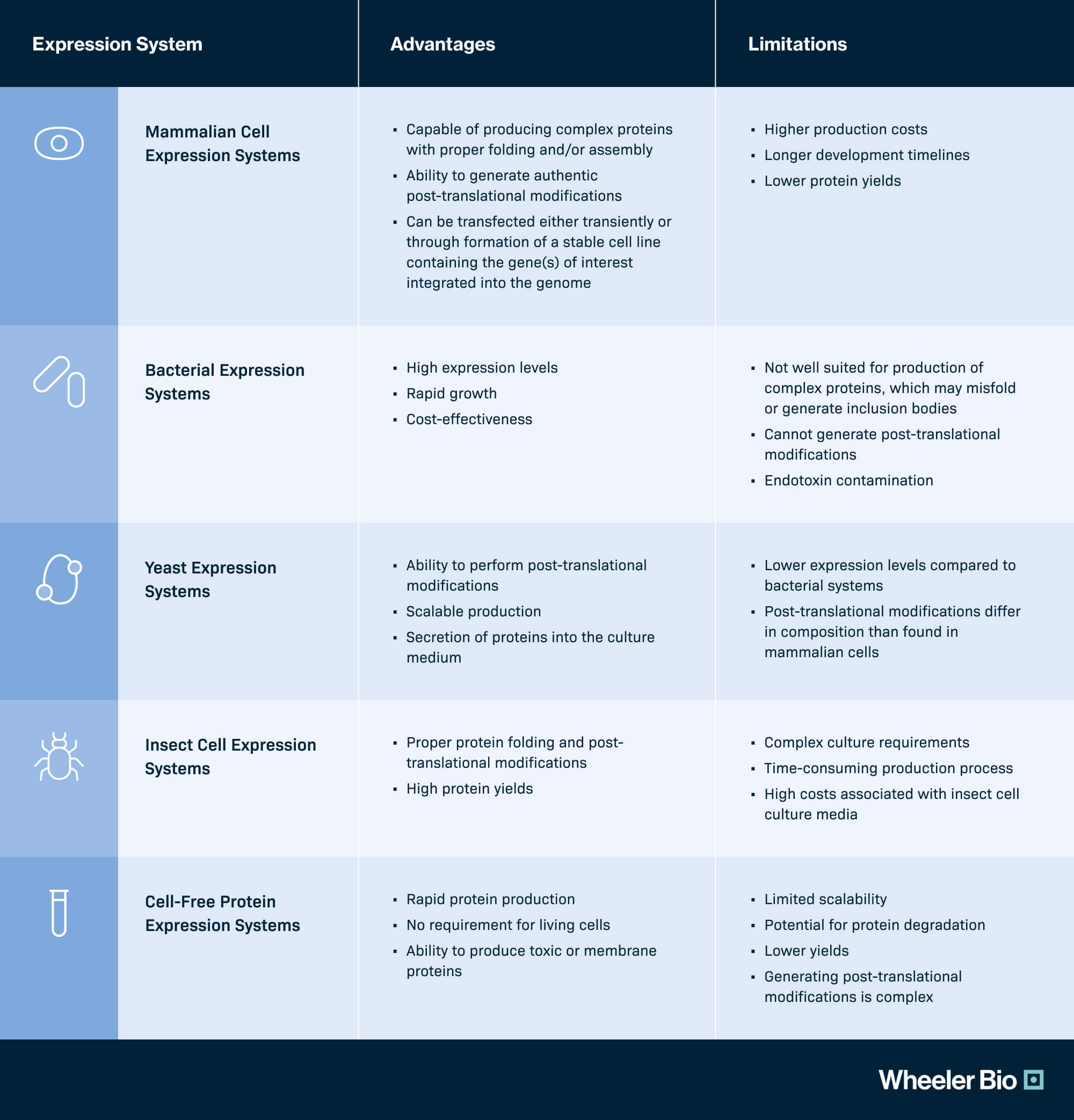
1. Mammalian Cell Expression Systems: Mammalian cells, including Chinese hamster ovary (CHO) and human embryonic kidney (HEK) cells are capable of producing complex proteins with proper folding and/or assembly and have the ability to generate authentic post-translational modifications. Mammalian cells can be transfected either transiently or through formation of a stable cell line containing the gene(s) of interest integrated into the genome.
2. Bacterial Expression Systems: Bacteria such as E. coli are widely used as expression hosts due to their rapid growth, well-understood genetics, and ability to express foreign genes; though are not particularly well suited for production of complex proteins, which may misfold or generate inclusion bodies, and do not possess the ability to generate post-translational modifications.
3. Yeast Expression Systems: Yeasts, including baker’s yeast and Pichia pastoris, have developed genetic systems, easy to culture, and can perform post-translational modifications, but these typically differ in composition than found in mammalian cells.
4. Insect Cell Expression Systems: Insect cells, such as those derived from the fall armyworm, Spodoptera frugiperda, Sf9 and Sf21, are used as expression systems to produce complex, properly folded proteins, including those requiring post-translational modifications. Recombinant protein production requires infection of cells by recombinant Baculovirus constructs to deliver the gene(s) of interest.
5. Cell-Free Protein Expression Systems: Cell-free protein expression systems traditionally use cell extracts from E. coli or wheat germ to produce recombinant proteins in vitro rather than using intact cells. These systems offer rapid protein synthesis, allow for easy manipulation of reaction conditions, and are useful for producing toxic or membrane proteins and high-throughput screening. Scalability of these systems is currently limited and generating post-translation modifications is complex.